Dr. David ReddyMedicines for Malaria Venture
Dr. David Reddy is CEO of the Medicines for Malaria Venture, a leading product development partnership in the field of antimalarial drug research and development.
An estimated 125 million pregnancies around the world are at risk from malaria annually.[1] For both mother and baby, the disease is potentially life-threatening and babies that do survive are often born with low birth weight, which is associated with negative impact on development.[2]
These mothers-to-be need medicines to both protect them from getting infected with malaria and to treat them should they fall ill. Yet, these needs remain inadequately addressed.
Medicines for Malaria Venture (MMV) is working with partners to improve the quality of global supplies of the World Health Organization (WHO)-recommended medicine sulphadoxine-pyrimethamine (SP) for use as intermittent preventive treatment in pregnancy (IPTp). As a partner in the Unitaid-funded Jhpiego-led consortium TIPTOP, MMV is supporting improved coverage of SP for IPTp in Africa through community health workers by strengthening the supply side for WHO-prequalified SP.
For women who become infected with malaria during pregnancy, WHO currently recommends artemisinin combination therapies (ACTs) for the treatment of malaria in the second or third trimesters, and quinine with the antibiotic clindamycin in the first trimester.
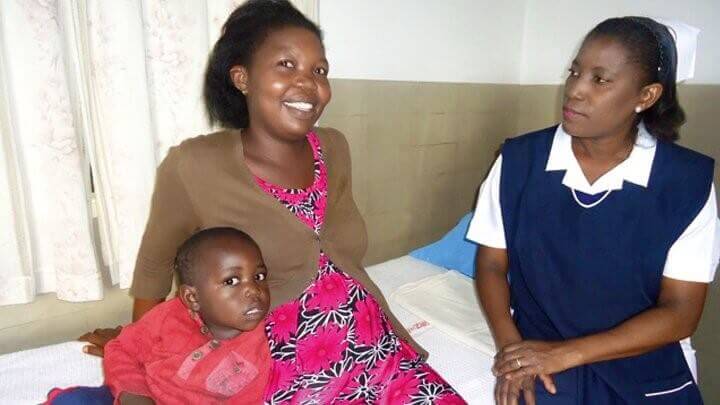 Angela Kangulumais is a primary school teacher in Zambia. She is 6 months pregnant with her second child. Angela is no stranger to malaria—she fell ill just before her first pregnancy and was treated with an ACT. Pregnant women at risk of malaria like Angela benefit from IPTp to protect themselves and their unborn babies from malaria. Photo: Medicines for Malaria VentureBased on meta-analyses comparing treatment outcomes using the ACT artemether-lumefantrine (AL) versus quinine in first trimester pregnancies and following a recommendation for policy change from an Expert Review Group, WHO is considering whether to recommend the use of all ACTs in all trimesters of pregnancy. That said, the data set regarding the use ACTs other than AL in the first trimester is comparatively small. MMV is working with the Liverpool School of Tropical Medicine’s Timika Research Facility in Indonesia to analyse historical data and to capture new evidence regarding the use of the ACT dihydroartemisinin-piperaquine (DHA-PQP) in the first trimester of pregnancy. These new analyses will contribute to the evidence base regarding safety and tolerability of this ACT during pregnancy.
Angela Kangulumais is a primary school teacher in Zambia. She is 6 months pregnant with her second child. Angela is no stranger to malaria—she fell ill just before her first pregnancy and was treated with an ACT. Pregnant women at risk of malaria like Angela benefit from IPTp to protect themselves and their unborn babies from malaria. Photo: Medicines for Malaria VentureBased on meta-analyses comparing treatment outcomes using the ACT artemether-lumefantrine (AL) versus quinine in first trimester pregnancies and following a recommendation for policy change from an Expert Review Group, WHO is considering whether to recommend the use of all ACTs in all trimesters of pregnancy. That said, the data set regarding the use ACTs other than AL in the first trimester is comparatively small. MMV is working with the Liverpool School of Tropical Medicine’s Timika Research Facility in Indonesia to analyse historical data and to capture new evidence regarding the use of the ACT dihydroartemisinin-piperaquine (DHA-PQP) in the first trimester of pregnancy. These new analyses will contribute to the evidence base regarding safety and tolerability of this ACT during pregnancy.
In addition to expanded use of ACTs for treatment in pregnancy, the malaria research community is also focused on exploring the use of DHA-PQP as a potential substitute for SP for IPTp in the future, should SP’s preventive efficacy decline. MMV is working with the London School of Hygiene and Tropical Medicine in Tanzania on a study to evaluate the cardiac safety of a single course of IPTp with DHA-PQP in pregnant women with and without asymptomatic infection.
For the future, we are also looking at the medicines we have in development and how they might be used for pregnant women. Traditionally, most drugs are tested for safety in pregnancy quite late in development (if at all), since in many therapeutic areas, a product that cannot be used in pregnancy can still have enormous therapeutic value. However, in the case of malaria, the needs of pregnant women are clear and compelling. MMV thus focuses early on in its drug development process to identify molecules that may have an acceptable safety profile in pregnancy. This focus includes conducting standard embryo-foetal development studies in parallel with other non-clinical safety assessment or phase I studies. A favorable safety report early on means that these molecules could be prioritized for development.
In addition, with MMV’s support, a review was carried out and published to help guide the prioritization of antimalarial drugs for development. It detailed the non-clinical safety of all non-artemisinin antimalarial drugs. [3] The article summarized data that was previously not easily accessible to health experts and is helping to guide the development of next-generation antimalarial medicines that could have an appropriate safety profile in pregnant women.
With our partners, MMV is committed to investigating all the avenues to improve pregnancy outcomes for women at risk of malaria. By focusing on protecting and treating pregnant women at risk, we safeguard two lives, not just one.
[1] Dellicour S et al. “Quantifying the Number of Pregnancies at Risk of Malaria in 2007: A Demographic Study”. PLos Med; 7(1): e1000221 (2010).
[2] Schantz-Dunn J & Nour NM. “Malaria and Pregnancy: a global health perspective”. Rev Obstet Gynecol; 2(3):186–192 (2009).
[3] Clark RL. “Animal Embryotoxicity Studies of Key Non-Artemisinin Antimalarials and Use in Women in the First Trimester.” Birth Defects Res. 109(14):1075-1126 (2017).