Emily ConronGHTC
Emily is a senior US policy and advocacy officer with GHTC managing congressional outreach, policy development, and legislative analysis to support the US advocacy work of the coalition.
On November 19, the Global Health Technologies Coalition (GHTC) convened its first virtual Hill event of the COVID-19 pandemic, connecting a group of congressional staff with representatives from four organizations advancing COVID-19 innovations to meet global needs. Twelve staffers representing members serving on key committees participated in the virtual dialogue. GHTC hosted the conversation to continue educating allies on the Hill about the need for COVID-19 innovations designed for use in low-resource settings, and how the US Agency for International Development (USAID) could support that effort—if Congress appropriates sufficient emergency funding in the next COVID-19 relief bill. The organizations who participated have strong records of advancing innovations for health emergencies with USAID support and are poised to play an important role in the global response to COVID-19.
The event began with a presentation by Dr. Rebecca Richards-Kortum, director of the Rice 360° Institute for Global Health, on the institute’s COVID-19 diagnostic tool. The simple-to-use, rapid point-of-care diagnostic offers results in 30 minutes or less, using a platform that is expected to cost less than US$5,000 per instrument and less than $2 per test. With USAID support, Rice is validating the test and plans to pursue emergency use authorization from the US Food and Drug Administration (FDA). The team hopes to scale the test quickly to countries in sub-Saharan Africa where they have research partners and infrastructure in order to make an impact in communities where trained laboratory personnel, basic hardware, and reagents are not readily available to support widespread testing using currently available COVID-19 diagnostics.
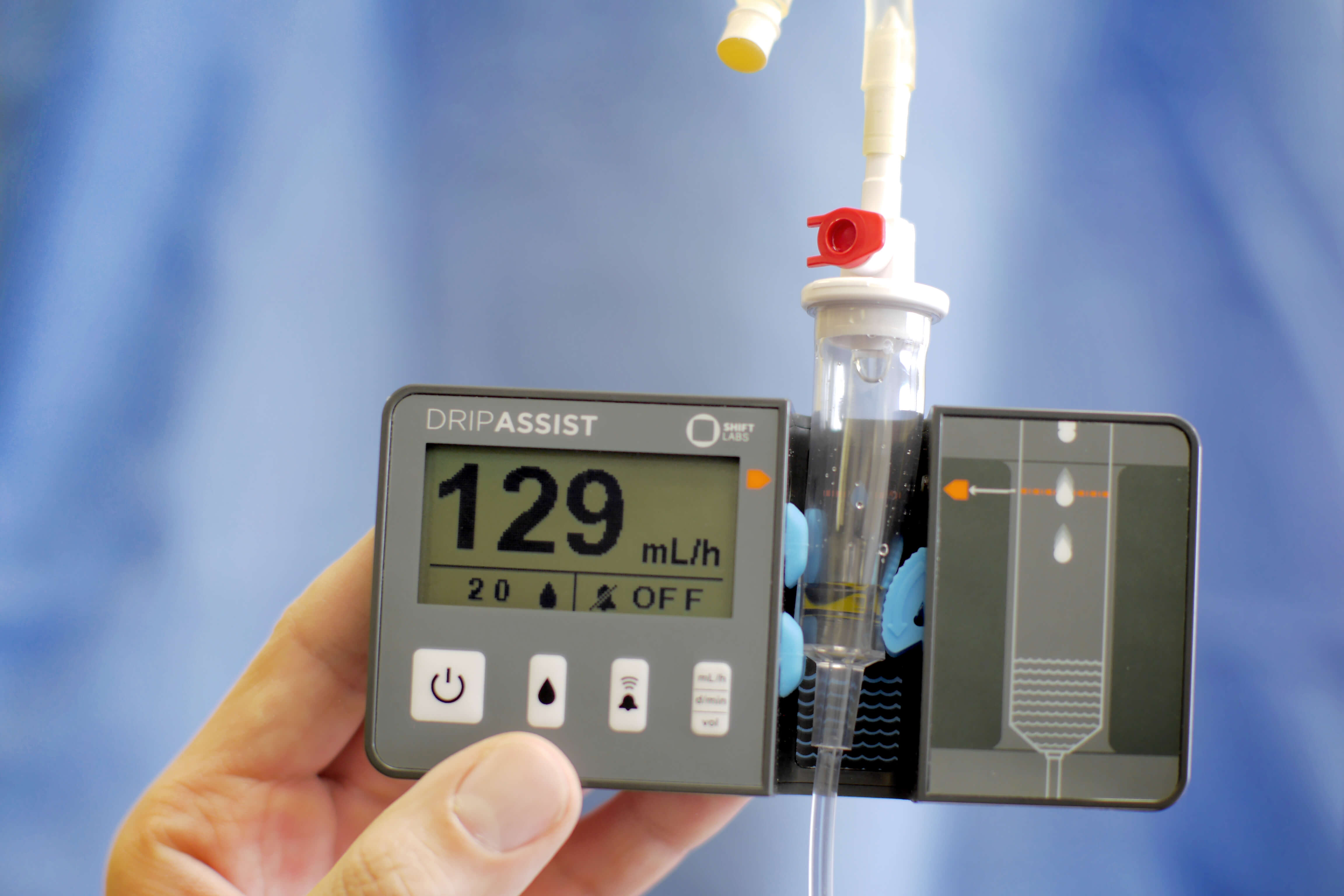
Next, Dr. Beth Kolko, co-founder and CEO of Shift Labs gave an overview of its DripAssist IV device. This ingenious device, designed to be durable, portable, easy to assemble, and operable with minimal training, can help a clinician anywhere in the world give IV medication safely and effectively without the use of costly infusion pumps. DripAssist, which was developed with support from USAID through the Fighting Ebola and Saving Lives at Birth Grand Challenges, was widely used during the recent Ebola outbreak in the Democratic Republic of the Congo—and even more recently was deployed in health care facilities in the United States facing a shortage of infusion pumps to address a surge in COVID-19 patients. “Designing for global needs gives engineers like our team a set of constraints that requires them to be more inventive,” Dr. Kolko noted, “And on the back end, you end up with a device that is more widely deployable,” including right here at home.
Dr. Devin Sok, senior director of antibody discovery and development at GHTC member IAVI, followed with a presentation on IAVI’s COVID-19 antibody research efforts and how emerging antibody therapies could be a key tool to defeat COVID-19 globally. IAVI is focused on developing COVID-19 monoclonal antibodies both to treat individuals just exposed to the virus to stop progression to the severe form of the disease and also to prevent infection in individuals who might be at risk of exposure, like a temporary vaccine. While antibody therapies are typically expensive, Dr. Sok referenced the example of a low-cost monoclonal antibody product already on the market for rabies and noted that IAVI is proactively taking several steps in developing its product to drive down costs and ensure that it will be accessible and deployable in low-resource settings worldwide, even though this might delay product introduction. “We were aware from the beginning that we were not going to be first to market, but the priority was to be first to access,” he said.
 OraSure Technologies' rapid HIV self-test. Photo: World Health OrganizationFinally, Dr. Stephen Tang, president and CEO of OraSure Technologies, shared more about his company’s progress on developing a rapid antigen self-test for COVID-19, a tool with potential to dramatically simplify and expand access to COVID-19 testing in the United States and globally. Described by Dr. Tang as a “lab on a swab,” the test uses a self-collected nasal sample and delivers easy-to-read results at point of care without the need for additional instruments or laboratory analysis. OraSure’s history of designing diagnostics for global health needs has positioned the company well to support global access to COVID-19 diagnostic tools. It created the first rapid oral HIV self-test prequalified by the World Health Organization and the first FDA-approved rapid diagnostic test for Ebola, and it is now leveraging the same lateral flow platform these tests are built on for its COVID-19 diagnostic. Subject to receipt of an emergency use authorization by FDA, OraSure’s rapid self-test for COVID-19—developed with funding from the Biomedical Advanced Research and Development Authority—could be deployed worldwide. Reflecting on the lessons OraSure has drawn from its experience developing HIV diagnostics and what’s required to ramp up COVID-19 diagnostic testing to the massive scale the world requires, Dr. Tang emphasized several interrelated factors: instrument-free self-tests are ideal for countries with limited laboratory infrastructure; ease of use is key, particularly in countries with low literacy rates; experience counts when it comes to working with regulators, particularly during health emergencies; and the ability to scale manufacturing to meet demand is paramount for impact.
OraSure Technologies' rapid HIV self-test. Photo: World Health OrganizationFinally, Dr. Stephen Tang, president and CEO of OraSure Technologies, shared more about his company’s progress on developing a rapid antigen self-test for COVID-19, a tool with potential to dramatically simplify and expand access to COVID-19 testing in the United States and globally. Described by Dr. Tang as a “lab on a swab,” the test uses a self-collected nasal sample and delivers easy-to-read results at point of care without the need for additional instruments or laboratory analysis. OraSure’s history of designing diagnostics for global health needs has positioned the company well to support global access to COVID-19 diagnostic tools. It created the first rapid oral HIV self-test prequalified by the World Health Organization and the first FDA-approved rapid diagnostic test for Ebola, and it is now leveraging the same lateral flow platform these tests are built on for its COVID-19 diagnostic. Subject to receipt of an emergency use authorization by FDA, OraSure’s rapid self-test for COVID-19—developed with funding from the Biomedical Advanced Research and Development Authority—could be deployed worldwide. Reflecting on the lessons OraSure has drawn from its experience developing HIV diagnostics and what’s required to ramp up COVID-19 diagnostic testing to the massive scale the world requires, Dr. Tang emphasized several interrelated factors: instrument-free self-tests are ideal for countries with limited laboratory infrastructure; ease of use is key, particularly in countries with low literacy rates; experience counts when it comes to working with regulators, particularly during health emergencies; and the ability to scale manufacturing to meet demand is paramount for impact.
After the presentations, the congressional staff participants posed a range of thoughtful questions about the innovations as well as the innovators’ perspectives on what the United States can do support the global response to COVID-19—from how US military research capabilities can be leveraged to design COVID-19 products suited for austere settings, to how members of Congress who represent researchers and research institutions can better advocate for resources needed to support science, to the specific measures that must be taken to scale up COVID-19 diagnostic capacity in the United States and around the world.
As we consider the challenges of delivering certain COVID-19 products—including the leading vaccine candidates—everywhere they are needed worldwide, GHTC will continue to connect policymakers and their teams with innovators working to design tools with global access in mind. We hope this dialogue creates the foundation for an enduring partnership between congressional champions for global health innovation and the researchers whose work is fueling progress against COVID-19 and other global health threats.