Matthew RobinsonGHTC
Matthew Robinson is a policy and advocacy officer at GHTC who leads the coalition's multilateral advocacy work.
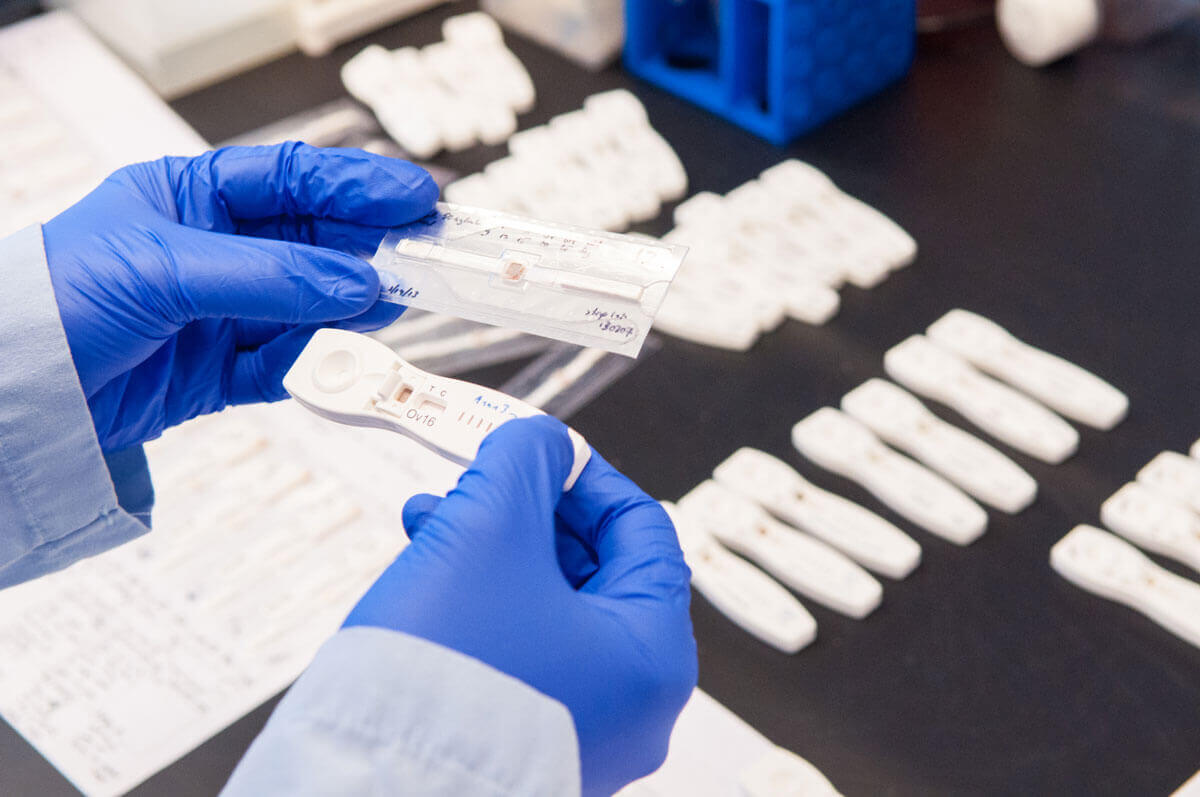 A researcher holds onchocerciasis (river blindness) diagnostic tests. Photo: PATH/Patrick McKernLast Thursday, the World Health Organization (WHO) took the first step toward establishing an essential diagnostics list (EDL), releasing a proposal outlining how it intends to develop and operationalize the list by early 2019. This move follows calls by GHTC and other partners for the agency to establish an EDL to provide guidance to countries on the vital diagnostics
that should be made available through health systems to complement WHO’s existing Model List of Essential Medicines (EML).
A researcher holds onchocerciasis (river blindness) diagnostic tests. Photo: PATH/Patrick McKernLast Thursday, the World Health Organization (WHO) took the first step toward establishing an essential diagnostics list (EDL), releasing a proposal outlining how it intends to develop and operationalize the list by early 2019. This move follows calls by GHTC and other partners for the agency to establish an EDL to provide guidance to countries on the vital diagnostics
that should be made available through health systems to complement WHO’s existing Model List of Essential Medicines (EML).
As GHTC has previously noted, the need for an EDL to complement the EML is clear and has been compellingly demonstrated. An essential diagnostics list would not only help health care providers, particularly in low- and middle-income countries, improve diagnostic capacity and access, and thus improve patient care, it also holds potential to catalyze research and development (R&D) for new diagnostics by exposing health areas where we today lack appropriate and effective tools. GHTC is pleased to see WHO heed this call from advocates and scientists and begin the process to establish an EDL.
 A researcher holds onchocerciasis (river blindness) diagnostic tests. Photo: PATH/Patrick McKernThe proposal released by WHO lays out the broad principles the agency will follow in standing up an initial version of the diagnostics list. This includes:
A researcher holds onchocerciasis (river blindness) diagnostic tests. Photo: PATH/Patrick McKernThe proposal released by WHO lays out the broad principles the agency will follow in standing up an initial version of the diagnostics list. This includes:
Moving forward, WHO’s essential medicines staff will consult with the Expert Committee on the Selection and Use of Essential Medicines at its meeting in March to gather technical input on how to best operationalize the principles outlined above. Based on this feedback, a pilot group of diagnostics will be evaluated based on existing evidence of programmatic impact, with the goal of completing the development of an initial version of the EDL by early 2019.
GHTC will continue to follow this issue closely and work with our members to ensure that the first version of the EDL is solidly grounded in our decades of combined experience with diagnostics and that the process of establishing the list moves forward efficiently. Keep watching this space for updates.